Uncategorized
New Choose: This Noninvasive Glucose Monitoring Answer Might Assist 422 Million Individuals
I fear loads about Mark, Liz and Julia. Mark was my flatmate in London in 1975, and we’ve stored in shut contact ever since. Liz and Julia are my sisters-in-law. All of them have diabetes. I already misplaced a pricey pal to diabetes final 12 months. I don’t need to lose one other one.
All three are very diligent about maintaining their sugar ranges beneath management. But it surely’s an enormous problem — and it’s costly. It prices $25 to $70 to take the usual complicated glucose check plus a health care provider’s workplace go to charge of $100 to $200. For those who’re utilizing strips, they value $100 to $150 for a month’s provide. And it includes pricking your finger. Blood sugar testing ache is a each day drudgery for a lot of diabetes sufferers.
Firms are conscious of this downside. For many years, dozens of firms — together with heavyweights like Abbott, Apple and Samsung in addition to many smaller ones you’ve by no means heard of — have tried to develop a extremely correct non-invasive answer. None have succeeded. The accuracy eluded them.
The hurdles — each technological and monetary — are daunting. JDRF CEO Dr. Aaron Kowalski has been knee-deep on this planet of rising diabetes know-how for many years. “There are main know-how challenges which might be so large, it’s important to marvel if there may be even a necessity anymore,” he stated.
He’s not alone in that evaluation. John L. Smith is among the main diabetes know-how consultants and creator of the seminal paper “The Pursuit of Noninvasive Glucose: Looking the Deceitful Turkey” article (first printed in 2006 and final up to date in a 2020 seventh version). He says that it will take a minimal of 5 years and $25 million to $30 million to get a product to market.
Given the immense technological and monetary challenges, I’d have wager that the primary firm to crack this know-how can be one of many larger legacy firms. However I’d have been improper.
As an alternative, it’s ViiT Well being — a small startup that’s tantalizingly near finalizing a glucose monitoring gadget that’s commercial-ready, noninvasive, extremely correct, low cost, simple to make use of and fast (leads to 15 seconds!). It’s flying by means of its medical trials in Mexico, with simply a few months to go. Then it should submit a request for clearance from the nation’s FDA-equivalent authorities company.
Like most different firms innovating within the noninvasive glucose monitoring discipline, ViiT Well being makes use of spectroscopy as the premise of its know-how.
Spectroscopy is the examine of the absorption and emission of sunshine and different radiation by matter. It includes the splitting of sunshine (or extra exactly, electromagnetic radiation) into its constituent wavelengths (which could be recognized by totally different colours).
Nearly all of firms attempting to develop a noninvasive answer (together with ViiT Well being’s potential opponents) are utilizing mirrored mild. The issue? Mirrored mild doesn’t “see” the total spectrum of colours. It solely sees one “analyte.” An analyte is solely a chemical substance that’s the topic of a chemical evaluation. So one analyte can be utilized to focus on glucose. However then it could possibly’t goal different chemical compounds.
ViiT Well being’s know-how makes use of direct transmittance of near-infrared mild mixed with its personal proprietary pc imaginative and prescient algorithms. It’s tougher to grasp this know-how. It took ViiT Well being 5 years. However now the corporate has the flexibility to “see” a number of analytes and the total spectrum of colours.
That is essential. ViiT Well being can use the identical gadget that measures glucose to measure ldl cholesterol, triglycerides and blood alcohol ranges.
In actual fact, its subsequent noninvasive monitoring product can be for ldl cholesterol. That’s an enormous market. Practically 94 million folks on this nation aged 20 or older have levels of cholesterol of over 200 (together with me). Each rattling time I see my physician, I’ve to take a blood check to see my newest levels of cholesterol. I’d welcome a noninvasive gadget, particularly one that would give me outcomes proper there after which within the physician’s workplace as an alternative of three to 5 days later.
The glucose monitoring market can be large. Worldwide, 422 million folks have been recognized with diabetes. 88 million People have pre-diabetes. And the scary half is that 86% of these 88 million don’t know they’ve it. 34 million People have diabetes. In Mexico, the place ViiT Well being’s two co-founders are from, 20 million dwell with diabetes. And the illness is the second-leading reason for demise within the nation.
It is a enormous alternative for the primary firm that will get noninvasive testing proper. Getting it proper means it have to be reasonably priced and supply extremely correct and quick outcomes.
ViiT Well being checks all three containers. It’s 5 occasions cheaper than customary complicated glucose checks and is 90% as correct. It’s 5% to 10% extra correct than fast checks. And also you get outcomes virtually instantaneously. It takes an ultra-speedy 10 to 15 seconds (versus 1.5 hours for the complicated checks).
ViiT Well being leads the pack by a major margin. CEO Luis Fernando says the corporate is at the least 5 years forward of all the competitors. 5 years is consistent with what John Smith says. However even when Luis is one to 2 years too optimistic, that’s nonetheless an infinite head begin. The truth that this firm has put appreciable distance between itself and heavyweights like MIT (which is utilizing laser pulses to observe glucose) is a formidable technological achievement. And it offers ViiT Well being stable defensibility.
The corporate is forging forward at full velocity, decided to leverage its first-to-market benefit. Its intelligent go-to-market technique targets Mexico as the primary nation it should start promoting in. If Mexican authorities clearance goes in accordance with plan, it’ll occur earlier than the top of the 12 months with gross sales to clinics, personal firms and hospitals instantly gearing up. The corporate initiatives it should acquire a bit greater than $eight million in gross revenue subsequent 12 months in Mexico.
Then ViiT Well being plans to pursue U.S. FDA clearance. Earlier than submitting an software, the corporate will want extra medical trials to display that it meets American security requirements and that its effectiveness is equal or higher than at present accessible merchandise. That stated, it normally takes three to 6 months to acquire 510(okay) approval.
It’s not a excessive bar, however we should be conscious there may be threat right here. I’d characterize FDA approval as a “excessive chance,” not a given. U.S. FDA protocols aren’t a lot totally different from Mexico’s. What’s extra, the knowledge and information that the corporate collected and submitted to the Mexican authorities for certification ought to facilitate the approval course of within the U.S.
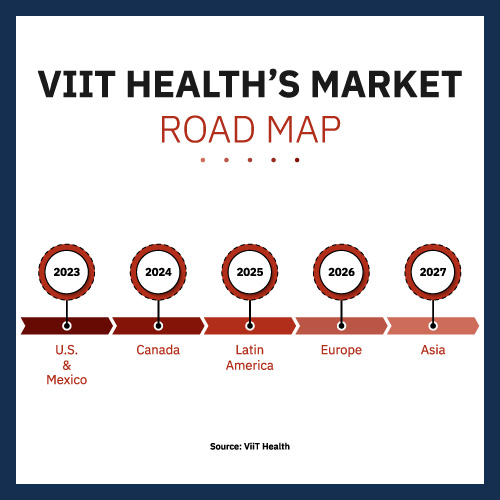
Given the corporate’s preliminary U.S./Mexico focus, Canada and Latin America are logical international locations for ViiT Well being to focus on two to a few years down the highway. And bear in mind, it will be doing so with no efficient competitors. Even its entry into Europe in 2026 needs to be with out severe competitors. By the point it hits Asia in 2027, it should have established itself as a globally trusted model. It’s doable that firms may develop comparable know-how by that point. Even so, they’d be at a definite drawback.
Whereas ViiT Well being’s funding profile is compelling, in its broad strokes it’s not that uncommon. Together with different progressive medtech firms, it affords promising however not fairly totally confirmed know-how. New options to massive, decades-old and intractable issues normally create immediate curiosity within the market, particularly when — as in ViiT Well being’s case — they’re 80% cheaper than present options.
ViiT Well being additionally has the wind at its again, because it’s promoting right into a fast-growing market. The blood glucose monitoring market is forecast to develop 9.6% yearly till 2026. And because the sole beneficiary of potential widespread adoption, ViiT Well being ought to expertise fast income development.
The investing alternative is excellent as is. However there’s much more to love. ViiT Well being is throwing into the combination a further two large bonuses. ViiT Well being can be restructured right into a holding firm with a purpose to soak up two different related applied sciences which have proven nice promise.
One makes use of pc imaginative and prescient to detect breast most cancers. The opposite makes use of pc imaginative and prescient to detect pores and skin most cancers. Validation of those two applied sciences is at a a lot earlier stage than ViiT’s glucose monitoring know-how. Mexican and U.S. authorities clearance is a few years down the highway. Following the playbook used for its glucose monitoring know-how, ViiT Well being would pursue authorities approval from Mexico first, then the U.S.
The extra value to develop these merchandise shouldn’t be an issue. Whereas it’s not fairly a performed deal but, a fund has mushy pledged to take a position $2 million into the brand new holding firm. It’s a candy deal for traders. You’re getting three extremely promising monitoring/detection applied sciences for the worth of 1.
The upside is extraordinarily compelling. In fact, there are at all times dangers we should take into account. So let’s have a look at every threat individually…
- Know-how/Regulatory Danger Half 1. It doesn’t matter when you or I feel the know-how works nice. It has to work effectively sufficient to get authorities clearance. The chance is low for Mexico and better for the U.S. However clearance in Mexico would give ViiT Well being a excessive ground, which properly enhances its excessive ceiling.
- Know-how/Regulatory Danger Half 2. Three applied sciences housed in a single firm diversifies threat. That’s an excellent factor.
- Manufacturing Danger. Manufacturing could be arduous. ViiT Well being has recognized websites in Mexico Metropolis and New Mexico and can start manufacturing later this 12 months. 3D printing will play an enormous function. The crew doesn’t have a high-ranking manufacturing specialist, so it’ll have to rent one quickly. There’s threat right here that shouldn’t be ignored.
- Competitors Danger. ViiT Well being has no severe competitors. Danger is minimal. It is a enormous plus.
- Advertising and marketing Danger. Badly wanted well being options that save lives and cash finally catch on and turn out to be extensively adopted. Nonetheless, ViiT Well being has to competently market its product. Mexico doesn’t fear me. Each founders are Mexican-born and CEO Luis has a protracted entrepreneurial observe document there. The U.S. worries me a bit bit. However the firm will be capable of apply lots of the classes discovered in Mexico to its U.S. advertising and marketing efforts. And that ought to assist immensely.
U.S FDA approval looms as the largest hurdle and threat. However remember that FDA clearance can be the gateway to a a lot greater valuation. Nailing manufacturing is an in depth second. Danger total grades out as extraordinarily cheap. And the upside may be very excessive. Luis deserves kudos for getting the corporate to the cusp of clearance, commercialization and severe income technology.
And the cusp is what you need. A 12 months in the past would have been too early to evaluate threat. Put up-cusp and the corporate’s worth could have sky-rocketed. The timing is nearly good.
Deal Particulars
Startup: ViiT Well being
Safety sort: SAFE
Valuation (cap): $15 million
Minimal funding: $100
The place to take a position: Wefunder
Deadline: February 28, 2022
Methods to Make investments
ViiT Well being is elevating as much as $2 million on this spherical of funding on Wefunder. You’ll have to join an account there when you haven’t but.
When you’re signed in to Wefunder, head over to the ViiT Well being elevate web page. Now enter the quantity you need to make investments and click on the crimson “Make investments” button on the right-hand facet of the display. The minimal funding on this deal is $100.
Dangers
This chance, like all early-stage investments, is dangerous. Early-stage investments usually fail. ViiT Well being may have to boost one other spherical of funding in a 12 months, if not sooner.
If it executes effectively, this shouldn’t be an issue. However that’s a threat price contemplating when investing in early-stage firms. The funding you’re making is NOT liquid. Count on to carry your place for 5 to 10 years. An earlier exit is at all times doable however shouldn’t be anticipated.
All that stated, I consider ViiT Well being affords a lovely risk-reward ratio.
The submit New Choose: This Noninvasive Glucose Monitoring Answer Might Assist 422 Million Individuals appeared first on Early Investing.
